In recent years, oil reserves have been steadily depleting, and oil is a major source of fuel production. Research into alternative energy sources is therefore becoming increasingly important. An alternative to oil is synthesis gas (mixtures of carbon monoxide-CO and hydrogen-H2). It is a raw material for the production of diesel fuel, lubricants, valuable chemicals, etc. The reaction that takes place is hydrogenation of carbon monoxide. It is extremely important for environmental protection that this production process does not lead to pollution. Cobalt and cobalt-palladium catalysts facilitate this process.
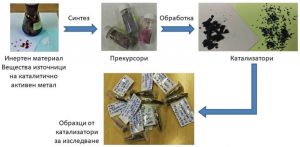
The ratio of the reactants carbon monoxide and hydrogen in the gas stream used and on the surface of the catalyst may be different, and this determines what products will be obtained. Various intermediate compounds are formed on the catalyst surface from the binding of carbon monoxide to cobalt, palladium and inert material. The formation of an alloy between the two metals varies the surface composition and changes the activity, selectivity and performance of the catalyst. A more complete utilization of the starting substances and their conversion into useful products is a quality of more selective catalysts. Here the production of undesirable by-products is reduced.

Apparatus used for studies of the composition of the catalyst surface and of the intermediate compounds
Optimization of the properties of catalysts involves changing the factors and adjusting the effect of their influence so that the catalyst is more active and has good selectivity to the desired product. Scientists from the Institute of Catalysis of BAS are working on this task within the project “Formation and evolution of the metal phase in supported cobalt-palladium catalysts for CO hydrogenation. Investigation of the relationship between CO/H adsorption ratio and selectivity”. The project leader is Assoc. Prof. Dr. Maya Shopska, and the funding is provided by the Bulgarian National Science Fund. The main objective of the development is to make a study on the sequence of occurrence, redistribution and depletion of intermediate compounds in the process by analyzing the CO/H adsorption ratio. In this way, the possibility of using it as an indicator to predict selectivity is verified. In the course of the studies, the researchers referred to a number of physicochemical analysis methods.
The influence of intermediate compounds and adsorption sites from the surface of catalysts on their performance in the process was obtained. The conditions under which the catalysts have the highest activity or best selectivity are determined.